Faculty Consulting
Supporting faculty in professional consulting opportunities.
Beginning January 5, 2026, the Faculty Consulting Program will transition from CU Medicine to CU Innovations. This change is designed to maintain the exemplary service currently provided while leveraging new tools and resources to improve efficiency, transparency, and deal quality for both faculty members and administrative staff.
We will support your consulting work with a streamlined, reliable process that makes required steps for external opportunities easier to manage and less cumbersome.
Please continue to use the existing CU Medicine workflow for new agreements and/or work with your current administrative staff for in-progress agreements. We are working through the transition and will notify you before anything changes.
This page will be updated as more information becomes available.
CU Anschutz Innovations was charged with prioritizing two things in this transition: maximize value and improve efficiency.
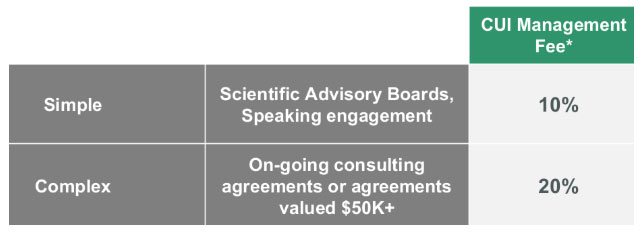
VALUE: We're excited to optimize revenue options with an updated fee structure: removing the Dean's 10%fee and instead implementing two categories:
1. Simple contracts, including but not limited to scientific advisory boards and speaking engagements, will incur a 10% management fee. We’re estimating that nearly 90% of agreements we receive will fall into this category. This is lower than the existing rate with CU Medicine.
2. Complex or large-scope contracts — including but not limited to ongoing consulting agreements, contracts that involve licensing and commercialization support to optimize faculty return, or include equity — will incur a 20% management fee. Each agreement is nuanced, but we expect only ~10% of all consulting contracts to fall into this category.
3. Note CU Anschutz Innovations will be trialing this new structure for one-year and it is subject to change. Agreements filed on January 5th or later through the new process (with Innovations and Ironclad) will be subject to these new rates. For existing agreements with CU Medicine, current rates will be honored and will not change.
EFFICIENCY
CU Anschutz Innovations is moving the non-clinical consulting agreement process from ContractLogix to Ironclad, a new contract lifecycle management software. This new platform offers:
1. Improved transparency & tracking
2. Better user experience
3. Customizable dashboards
4. Streamlined and automated approval tasks
Not Sure Where to Go for Your Consulting Activity?
Check out this guide:
| TYPE OF WORK | DESCRIPTION | EXAMPLES | PREVIOUSLY | FUTURE |
|---|---|---|---|---|
| CONSULTING AGREEMENTS | A faculty member is requested to give intellectual advice/opinion or providing support, but not patient care Does not apply to academic opportunities such as invited grant rounds, textbook or journal editorial roles | Speaker at an industry conference, advisory board member, research study advisor, product development consultant | CU Medicine | CU Anschutz Innovations *beginning January 5, 2026 |
| CLINICAL | A faculty member is providing patient care outside a managed care contract | UCH, UCHA, CHCO, DHHS, VA agreements, community clinic work, clinical lab agreements | CU Medicine | CU Medicine |
| MED/LEGAL | A faculty member is providing professional opinion for attorney, judge, or admin agency for an ongoing or contemplated judicial process | Testimony/expert at a trial, record review for a legal case, deposition, Independent Medical Exam | CU Medicine | Faculty/Department *change in effect as of October 1, 2025 |
| GRANTS AND CONTRACTS | A faculty member utilizing University facilities and equipment and conducted with financial and/or other valuable support from an external sponsoring entity | Includes any sponsored projects (both research and non-research), including all grants, as well as fee for service and revenue contracts. | Office of Grants and Contracts (OGC) | Office of Grants and Contracts (OGC) |
| CLINICAL TRIALS AND CDAS | A faculty member is engaged in industry funded research involving human subjects or is sharing confidential information with an external partner before a potential study | Industry sponsored clinical trial agreements involving human subjects, Confidentiality Disclosure Agreements (CDAs), Non-Disclosure Agreements (NDAs) | Clinical Research Admin Office (CRAO) | Clinical Research Admin Office (CRAO) |
Don’t see what you need in this list? For all contract types, click here.