Update on Clinical Research Activities
November 20, 2020
Dear Research Community Colleagues,
Changes to Clinical Research Activity: Effective December 1, 2020
In April of 2020, CHCO, UCHealth, all Anschutz Medical Campus CTRCs, and CU Anschutz collaboratively developed a Priority Ranking of Clinical Research by Grouping. This grouping guided the Campus COVID-19 Research Reconstitution plan based on the potential for direct benefit compared to risk profile of studies to participants.
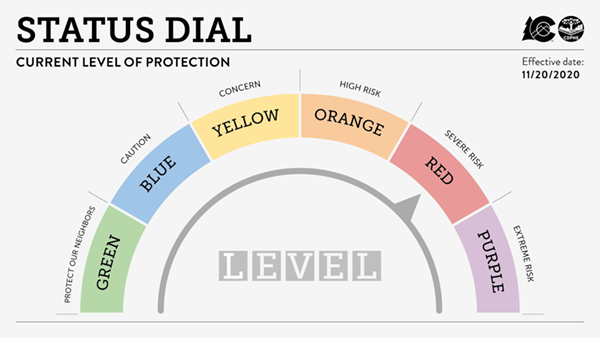
The State of Colorado & Colorado Department of Public Health and Environment (CDPHE) has issued a recent change to the COVID-19 status dial. Effective November 20, 2020, the State of Colorado is moving a number of counties to the RED – Severe Risk on the updated Status Dial, which has stricter COVID-19 restrictions to slow the rapid spread of COVID-19. The seven counties (Adams, Arapahoe, Boulder, Broomfield, Denver, Douglas, Jefferson) from the Denver metro area are now designated Red (Severe Risk) on the Dial.
In response to the public health guidelines for Red status for the entire Denver Metro area, CHCO, UCHealth, all CTRCs, and CU Anschutz will implement restrictions on the activity of some clinical research activitieseffective December 1, 2020. This will be done using the previously defined categories in the Priority Ranking of Clinical Research by Grouping.
For risk status ORANGE – High Risk – the institutions will limit the opening of new studies in Group 5 (5a, 5c, 5d, and 5e).
With the new status level of RED – Severe Risk, all prior restrictions will continue and all face-to-face research activities in studies in Groups 3d, 3g, 3h, 4b, 4d, 4e, 4f, and 5b will also be paused.
Note: Studies in Groups 3d, 3g, 3h, 4b, 4d-f and 5a-e that move ALL research activities to remote work, can continue research activities remotely.
Each institution recognizes the potential for exceptions to this guidance on a case-by-case basis. Requests for exception must provide a compelling
argument about why the study should remain open for face-to-face research visits despite Severe Risk status and how the study would comply with current state and local public health requirements. Please submit through the following mechanisms:
- CU Anschutz, CTRC, and CHCO: AMC Research Reactivation Survey
- UCHealth: Draft an email to [email protected] with
the following:
- IRB number and justification of why the study should remain open
For more information please visit COVID-19 clinical research activity updates.
Sincerely,
Thomas Flaig, MD
Vice Chancellor for Research